The Virgin Earth Challenge:
Remove one billion tons of carbon per year
from earth’s atmosphere.

The Virgin Earth Challenge:
Remove one billion tons of carbon per year
from earth’s atmosphere.
The Virgin Earth Challenge Terms and Conditions Agreement,
which is 14 pages of partly technical but mostly legal stuff, can be viewed at
http://www.virginearth.com/wp-content/uploads/2012/09/Virgin-Earth-Challenge-TsCs.pdf
Virgin Management Limited, the judges, can be reached at earth.challenge@virgin.co.uk
December 24, 2014
http://tinyurl.com/zw22yjy
December 22, 2014
Virgin Earth Challenge
The School House
50 Brook Green
London W6 7RR
United Kingdom
Dear Virgin Earth Challenge Judges,
In response to the Virgin Earth Challenge competition, here is my proposal for removing CO2 from earth's atmosphere without disruption to ecological well-being.
The "machine" to remove CO2 from the Earth's atmosphere already exists. It is called the ocean. Our task is to cleverly utilize the ocean’s removal service in a sustainable way. When one considers the magnitude of the task, what possible human contraption could be commensurate? Even if such device or infrastructure were mass-produced, with units placed at every unoccupied spot? Only the ocean, with its 360,000,000 km² of area, is a worthy interface agent.
Prior to the fossil-fuel age, the ocean received from the atmosphere 800 million tonnes per year of CO2. The mechanism was river water carrying the products of natural rock weathering. Those specific products were, and still are: a) Calcium; and b) Hydrogen carbonate ions (HCO3- ). Every HCO3- ion has been created by grabbing and removing a CO2 molecule from the air. Figure 1 illustrates this weathering process.
The guy from Virgin Atlantic Airways, Richard Branson, issued the Virgin Earth Challenge in 2007. An offer of prize money for devising an effective way to remove carbon from the atmosphere.
I had never heard of the Virgin Earth Challenge - VEC - until a recent telephone conversation with my good friend James Lewis about Naomi Klein's new book:
THIS CHANGES EVERYTHING: CAPITALISM VS. THE CLIMATE.
James was reading Klein's book and recommending me to do the same so we could discuss certain of Klein's topics from a common knowledge base.
James' advice is always good, so I got busy. In Chapter 7, titled NO MESSIAHS: THE GREEN BILLIONAIRES WON’T SAVE US, there was all the dish about Branson, Al Gore, Bill Clinton, etc., and describing the prize for removing and safely sequestering one billion tons per year of carbon (carbon dioxide?) out of earth's atmosphere. The purpose being, of course, that then we can keep flying in airplanes.
I have taken the lime-in-the-ocean idea, which has been put forward as remediation for ocean acidity, and adapted it to Branson's Virgin Earth Challenge. Here is my proposal.
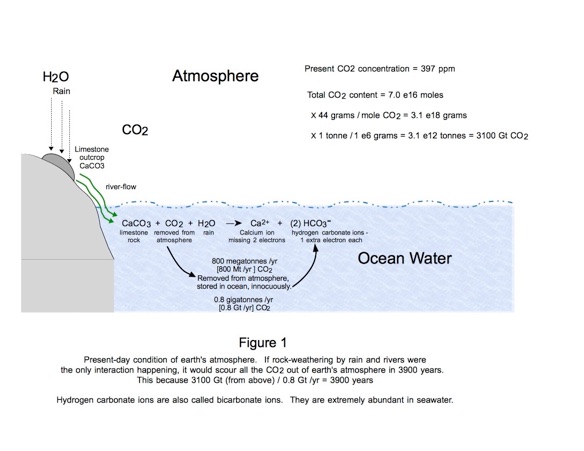
Here is a potent concept: If natural rain-induced limestone rock weathering were working in isolation it would rapidly (in the geologic sense) deplete the atmosphere of all its CO2. How rapidly? In about 3900 years, estimated.
Of course that would be a disaster because we are dependent on CO2 to maintain earth as warm as it is: 15 deg C. But hold that thought about limestone-rock weathering and the ocean. It contains the germ of an idea that will save the ecosphere when we humans cleverly intervene to manipulate limestone chemistry.
The air-water system before fossil fuels
Limestone rock weathering did not strip the earth's atmosphere of its CO2 because it does not work in isolation. There are two related effects always occurring at the air-water interface - the sea surface. They are: a) Seawater is constantly outgassing (OG) its free-dissolved CO2 molecules, stimulated by wind and wave action; and b) Air is constantly dissolving (DS) its free CO2 molecules, likewise by wind and waves. The relative rates of OG and DS are determined by the relative concentrations of water-dissolved CO2 and airborne CO2. Those concentrations affect molecule transfer through the air-water interface via the medium of CO2 partial pressures. More about that later.
These three effects, weathering (WT), outgassing (OG), and dissolving (DS) are illustrated in Figure 2. The numeric values are our informed estimates of the chemical conditions that prevailed pre-industrially.
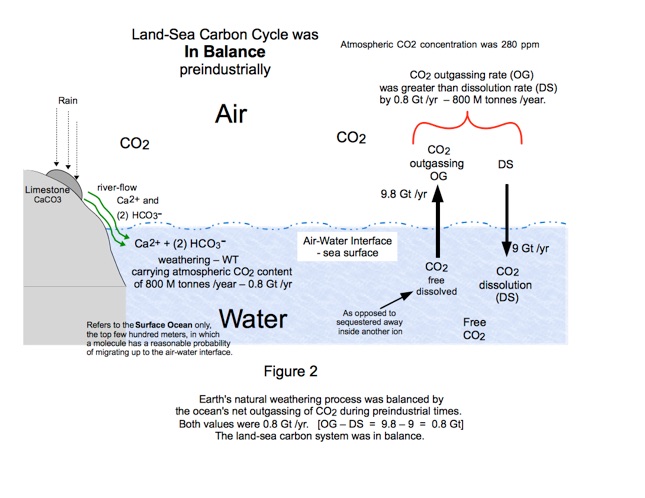
So there were three CO2 transfer mechanisms at work. The WT mechanism was moving CO2 out of the atmosphere into the ocean at a rate of 0.8 Gt / year. The OG and DS mechanisms, working against each other, were moving CO2 out of the ocean back into the atmosphere at the same rate, 0.8 Gt / year. OG exceeded DS. The air-water CO2 system was in balance.
The air-water system after fossil fuel industrialization
Human burning of hydrocarbons, coal, petroleum, and methane natural gas, has introduced a fourth CO2 mechanism into the earth system. It is represented by the coal smokestack in Fig. 3.
Response to Virgin Earth Challenge
ABSTRACT
A method for removing a substantial amount of carbon dioxide from earth's atmosphere is proposed in response to the 2007 Virgin Earth Challenge - VEC - which solicits ideas to remove and sequester 1 billion tonnes (1 Gt) per year of CO2 “without countervailing harmful effects".
The general supposition is a device acting directly within the atmosphere. However, the proposed method functions in earth's oceans in order to derive advantage from the large interactive interface between atmosphere and sea surface, an area of 360,000,000 square kilometers. It is felt that such a large junction area enables the water-to-air interface to outperform any electromechanical device that could be placed within the atmosphere proper.
The proposed method induces a sequence of chemical reactions in seawater which results in the removal and sequestration of free-dissolved CO2 molecules into carbonate ions, HC03- . Carbonate ions are superabundant in seawater, the consequence of eons of natural rock weathering upon land. The great majority of them remain in water solution indefinitely.
As free-dissolved CO2 molecules are removed from the ocean the CO2 partial pressure in water becomes lower than the CO2 partial pressure in the air above. The vast area of the air-water interface then becomes a route for atmospheric CO2 to dissolve in water, thereby escaping from the atmosphere. This process will continue for as long as human intervention continues inducing the chemical reaction sequence, which is:
ON LAND: CaCO3 —➤ CaO + CO2 [calcium oxide powder] Eq. (1)
(a) (b) (c)
(a) limestone - calcium carbonate;
(b) intense heat for reaction: 900-1200 deg C
(c) this CO2 must be captured and sequestered in basalt bedrock or caverns
IN WATER: CaO + H2O —➤ Ca(OH)2 [spontaneous in the sea] Eq. (2)
IN WATER: Ca(OH)2 + 2CO2 —➤ Ca(HCO3)2 [spontaneous] Eq. (3)
(d) (e)
(d) these two CO2 molecules are removed from the water
(e) those two CO2 molecules are sequestered within the two HCO3 ions
The sequence begins with high-temperature dissociation of limestone [Eq. (1)]. This act is the first step in the process of cement manufacture, a worldwide industrial activity for which humanity now mines 20 billion tonnes per year of limestone. It is proposed to divert worldwide coal-mining effort to expand limestone mining, which would produce an estimated additional 25 billion tonnes, 25 Gt.
The Eq. (1) through Eq. (3) sequence yields a ratio of 88 mass units CO2 removed from water per 100 mass units CaCO3 dissociated on land: that is, 88 /100.
Applied to 25 Gt CaCO3, the 88 /100 ratio gives 22 Gt CO2 removed from the sea. The amount removed annually from the atmosphere will be less than 22 Gt because the partial pressure decrease in air cannot keep pace with the partial pressure decrease in water as CO2 disappears from both sides of the interface. But the amount removed from the atmosphere surely will be much greater than 1 Gt.
This proposal envisions utilizing near zero-carbon energy to perform the following activities: 1) Mining; 2) Grinding; 3) Transport to lime kiln; 4) High-temperature dissociation [Eq. (1)]; 5) Transport of calcium oxide CaO to sea harbor; 6) Dispersal of CaO powder [Eq.(2)] at sea by ship.
To achieve near zero-carbon emission, all energy inputs - thermal, electric, and portable engine fuel - are to be derived from Generation-4 nuclear technology, specifically Liquid-Fuel Thorium Reactors - LFTR.
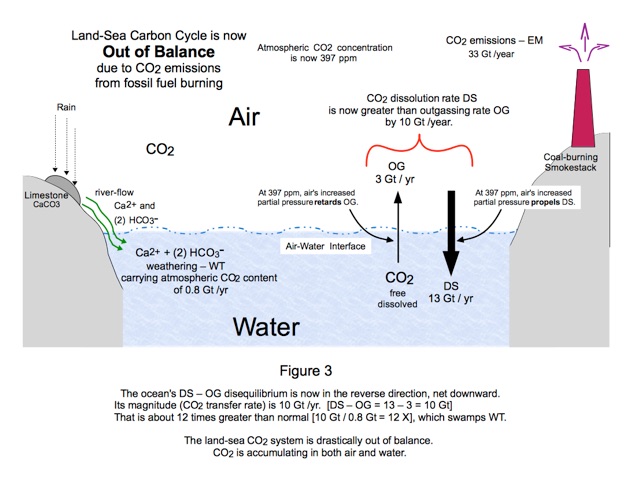
CO2 emissions, symbolized EM, have been accumulating for several centuries, so have increased the CO2 content of both atmosphere and surface ocean. The atmosphere more drastically: an approximate 40% increase (397 ppm / 280 ppm = 1.42). The surface ocean less so.
These relative accumulations have increased the downward DS movement and retarded the upward OG movement, as portrayed in Figure 3. The CO2 partial pressures of air and water are at work here. Today the net transfer through the air-water interface has reversed. Whereas it used to be
0.8 Gt / yr upward, now it is 10 Gt / yr downward.
That net 10 GT / yr swamps the continuing WT contribution of 0.8 Gt / yr. The air-water system has become drastically out of balance. The system is seeking a new physical / chemical equilibrium condition, but that is futile because the 33 Gt EM has not stopped and continues to cause yet more accumulation.
But here is the salient thing: The ocean is trying to clean our atmosphere. It is working on our behalf, pulling 10 Gt / yr CO2 out of earth’s atmosphere. The ocean’s removal rate is swamping the trifling contribution of natural limestone WT removal. It's a shame that the ocean is ruining itself in trying to help us, terrestrial life, but that's a separate issue for now.
The question for us is this: Is there some way that we humans can intervene in the four-mechanism situation of Figure 3? That is, can we intervene in such a way that we keep the ocean working beneficially for us
in the near term, yet not allow it to accumulate so much free dissolved CO2 that its increasing partial pressure weakens the downward DS effect in Figure 3? While also protecting the ocean from its self-destructive behavior?
I assert that such an intervention is possible. It is within our reach.
For ease and clarity of discussion, Figures 2 and 3 do not take into account the biological effects of sea plankton forming shells to support the food chain of vertebrate marine life. The chemical reaction sequence relating to plankton causes a portion of the CO2 that was sequestered in HCO3- to be released back into seawater solution. It thereby introduces an extra event into the air-water system which I have ignored in those two figures.
The ocean acidification problem is a separate issue from our atmospheric CO2 reduction challenge, as stated above. Nevertheless, for completeness, here is described the sea plankton reaction sequence: It begins with two of the superabundant HCO3- ions dissociating spontaneously
2 HCO3- —➤ 2CO32- + 2H+ Eq. A
The plankton then combine one calcium ion with the products of Equation A.
Ca2+ + 2CO32- + 2H+ —➤ CaCO3 + CO2 + H2O Eq. B
seashell compound, released into
the same as limestone free solution
This biochemical sequence is being disrupted by acidification, which is itself a consequence of too much CO2 free-dissolving into the surface ocean by the DS arrow through the air-water interface. Ocean acidification will be halted and corrected over time by implementation of my intervention plan.
Intervention by us
Revisit the limestone weathering phenomenon of Figure 1. We see CaCO3 breaking apart in a chemical reaction and thereby removing one CO2 molecule from the atmosphere’s inventory. Is there another way that CaCO3 can be encouraged to remove CO2 molecules?
Yes, there is a way. But this alternative reaction removes CO2 from seawater's inventory, not from the air’s inventory. It therefore tends to reduce the CO2 partial pressure on the water side of the air-water interface, thereby further encouraging the large reverse disequilibrium shown in Figure 3. That is to say, by removing free CO2 from the ocean we can keep the process running, in which the process is a "machine" that is removing net 10 Gt / yr CO2 from our atmosphere.
Think of it this way: As fast as the DS down-arrow pushes CO2 molecules out of the air and into the water, we have a CaCO3 reaction up our sleeve that immediately takes those same molecules out of the water. Thus the DS CO2 will not accumulate in the water, and it's partial pressure will remain lower than air's partial pressure. Our ocean machine will continue aiding the atmosphere and us land creatures, and over time the ocean itself will start to heal. All this will happen if we intervene to use CaCO3 to get rid of free dissolved CO2 as fast or faster than the DS-OG disequilibrium is putting it into the water.
Here is the limestone chemical reaction sequence that can achieve these benefits.
On land: CaCO3 —➤ CaO + CO2 calcium oxide plus a new CO2 (groan) Eq. (1)
intense heat
900-1200 °C
In water: CaO + H2O —➤ Ca(OH)2 calcium hydroxide Eq. (2)
In water: Ca(OH)2 + 2CO2 —➤ Ca(HCO3)2 calcium bicarbonate Eq. (3)
Two free dissolved molecules -
the target of our intervention
Calcium bicarbonate Ca(HCO3)2 from Equation 3 is a benign molecule already extremely abundant in seawater. It is so abundant that it already contains more than 100 times as much elemental carbon as does the culprit that we are chasing - CO2.
There is a very important result tucked away in the above reaction sequence. Namely, we have removed two CO2 molecules from the water while creating only one new CO2 molecule on land.
We are ahead by one molecule in our quest to reduce the total amount of free CO2 residing in the ecosystem. We're winning!
Those two CO2 molecules have been "sequestered" within the two hydrogen carbonate (bicarbonate) HCO3 ions that are bonded to the calcium atom (or Mg atom). Ocean chemists believe that superabundant bicarbonate molecules already hold about 87% of all the inorganic carbon that exists in the surface ocean. My proposal is to raise that percentage to 88%, speaking glibly.
Reactions (1), (2), and (3), and the industrial practices that accomplish them, require a lot of discussion. Before I begin that discussion allow me to say some important words about that new land-origin CO2 molecule that appears in Equation (1).
Carbon capture and sequestration - CCS
Point-source carbon dioxide emissions are possible to capture and sequester - Carbon Capture and Sequestration. It's expensive, but it is possible. We usually think of sequestration in terms of pumping the CO2 gas into an underground cavern. The weaknesses of the cavern idea are:
1) Caverns aren’t always conveniently located when we get serious about large-scale CCS; and
2) Caverns sometimes leak, with the CO2 making its way back into the ecosphere, usually groundwater.
A better idea is to sequester the carbon dioxide in basalt rock, which is the planet's most abundant bedrock. Basalt rock contains high concentrations of calcium, magnesium, and iron oxides which can ionically bond with CO2 molecules. Once bonded, that CO2 is retained over geologic time - forever, from the human standpoint.
As depicted in Figure 4, basalt sequestration is accomplished by bubbling CO2 into fresh water, then pumping that water into the interior of a porous basalt deposit, either terrestrial or beneath the seabed. This is illustrated in Figure 4. It is more expensive than cavern storage but it has the two advantages of scaleability to widespread use, and security from escape.
In any case, overall CO2 disposal expense is dominated by the capturing process, not the storage procedure. So the basalt method's cost is not crucial.
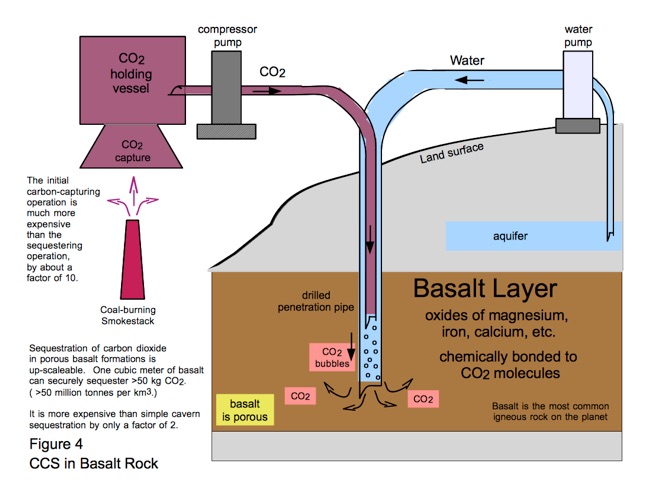
After basalt-storage infrastructure is scaled up, a desirable goal anyway for all the world's point-source CO2 emitters (which account for about 80% of the 33 Gt value in Fig. 3), we will be justified in claiming that the above chemical Equations (1), (2), and (3) make us two molecules ahead, not just the one CO2 molecule that we had earlier claimed. We're winning even faster!
Performing the intervention
Now the discussion that was promised concerning the industrial practices for Equations (1), (2), and (3).
Equation 1, the thermal decomposition of CaCO3, is the first step in cement manufacture. It is a common process performed in what is called a lime kiln. It requires concentrated heat to achieve a high temperature - in the 1000°C range. We're going to need a lot of thermal energy to carry this out on a large scale. It must be CO2 emission-free thermal energy in order for us to remain two molecules ahead. Duly noted.
To obtain CaCO3 we must mine limestone, then pre-grind it, then transport it to a lime kiln, which will be located near a basalt rock deposit. Mining requires wheeled and tracked vehicles and other equipment, all of which burn portable fuel. Grinding requires electricity. Railroad engines burn portable fuel. Pumping fluid into a penetrated rock formation requires electricity.
So we are going to need a lot of electricity and portable fuel, both of them also carbon-free like the thermal energy above, in order to remain two CO2 molecules ahead in our intervention. Also duly noted.
CaO in Equations (1) and (2), called quicklime, is a solid powder under standard conditions. We must transport that powder to a sea-harbor and load it aboard ships. The ships will be equipped with a discharging apparatus for dispersing the powder into the surface ocean at a calibrated rate. When the CaO disperses into seawater, chemical reactions (2) and (3) occur spontaneously. Equation (2) gives calcium hydroxide Ca(OH)2. In Equation (3) one molecule of Ca(OH)2 captures two molecules of free CO2 and "hides" them in HCO3- ions.
Shipboard engines imply even more carbon-free portable fuel, the same as the mining and land transport vehicles.
We will continue this intervention for years, while monitoring the seawater chemistry and adjusting our CaO dispersal. For every CaCO3 molecule that we decompose within a lime kiln, we eliminate two CO2 molecules from earth's ecosphere; that's the bargain.
The ocean "machine" keeps working at repairing our atmosphere of Figure 3. Meanwhile the ocean itself is healing, since those two CO2 molecules of Equation (3) are being taken directly out of dissolution in the ocean water, then sequestered within Ca(HCO3)2 molecules. Thus limestone intervention also remediates ocean acidification, by a different set of chemical reactions.
The rate of CO2 removal is naturally gradual and easily controllable, with no danger of overshoot.
Time-Frame
I will attempt to quantify my intervention plan to arrive at a time estimate for atmospheric recovery. My calculations will be based on the following three assumptions:
1) All coal mining on earth will cease. The equipment and personnel formerly committed to that industry will be repurposed to limestone mining.* (See remark at conclusion of Time Frame section.)
Not only will coal mining cease, but combustion of gaseous methane and petroleum for electricity generation also will cease. Petroleum for transportation will take many decades to phase out, certainly. But aside from that, petroleum and natural gas hydrocarbons will be reserved solely for production of fertilizers, plastics, fibers, medicines, and other essentials.
2) All remaining point-source carbon dioxide emissions, including the CO2 produced by our own limestone intervention reaction, Equation 1, will be captured and sequestered. Between these first two assumptions therefore, the 33 Gt / yr value for EM in Figure 3 will decline wonderfully.^
3) The three energy forms required for intervention, namely thermal, electric, and portable fuel, will themselves be carbon-free. I will have a lot to say about this later.
_______________
^ Large point-source emitters account for about 80% of global CO2 emissions, about 26 Gt / yr (33 Gt x 0.80 = 26 Gt). To sequester that annual amount into basalt at the 50 Mt per km3 rate in Fig. 4 would require
26 e9 tonnes x 1 km3 / 50 e6 tonnes = 520 cubic kilometers.
This is only an area 100 km by 100 km multiplied by 52 meters thickness. Not a very big piece of the planet.
_______________
Regarding our access to limestone resources, I will suppose that repurposing mankind's coal mining activity will enable us to extract an equal volume of limestone. This is perhaps simplistic since it ignores the difference in hardness between limestone and coal, and also ignores their different deposit locations - their closeness to the land’s surface. I imagine that the hardness comparison disfavors limestone’s accessibility, but its concentration nearer the surface will favor its accessibility. So perhaps the two aspects will cancel each other.
COAL: The amount of coal mined worldwide in 2013 was 7.5 billion tonnes, 7.5 e9 t.
At an average coal density of about 800 kg/m³, that coal's volume is given by
7.5 e9 t x 1000 kg / t x 1 m3 / 800 kg = 9.4 billion cubic meters, 9.4 e9 m3.
LIMESTONE: As stated above, suppose that our repurposed companies can provide us with that same volume of limestone, namely 9.4 e9 m3. With CaCO3 density about 2700 kg/m³ (much denser than coal), that equal volume will have a mass
9.4 e9 m3 x 2700 kg / m3 = 25 e12 kilograms or 25 billion tonnes, 25 e9 t of limestone per year, available for use in our intervention.
My calculation takes no account of CaCO3 richness content in limestone-bearing ore (but do also include MgCO3), so it is a bit simplistic.
In chemical reaction equations, the actual quantities of individual compounds are expressed in units of moles. Every specific chemical compound has a certain determined mass, expressed in grams, per mole. That value is called molar mass, or molecular mass.
When a chemical reaction is carried out at a real physical scale, the number of moles of a particular constituent compound in the equation is equal to the number of molecules that appear in that equation. So for equations (1), (2), and (3) in our CaCO3 intervention sequence, one mole of CaCO3 eliminates two moles of CO2. This 1-to-2 relation can be expressed in macro physical quantities (industrial mass, or volume), by referring to the molar masses of CaCO3 and CO2. They are
CaCO3 100 grams / mole
CO2 44 grams / mole
Since one mole of CaCO3 removes two moles of CO2,
1 x 100 g CaCO3 removes 2 x 44g CO2 = 88 g CO2.
This can be expressed as a proportional fraction, in either way:
100 g CaCO3 / 88 g CO2 or 88 g CO2 / 100 g CaCO3.
That is, we can use either fractional expression for calculation purposes.
_______________
The total aggregate amount of CO2 in the earth's atmosphere is known with good confidence. It is 3.1 trillion tonnes, equal to 3.1 e15 kg, equal to 3.1 e18 grams.
Per the calculation above, humanity’s coal-to-limestone mining switchover will provide us with 25 e9 tonnes / yr CaCO3. In gram units, 25 e15 g / yr. Therefore the amount of carbon dioxide that that limestone can remove from the ocean is given by
25 e15 g X 88 g CO2 / 100 g CaCO3 = 22 e15 g CO2 / yr
removed from the ocean by our intervention.
As explained, that quantity of CO2 will be coming out of the ocean, not from the atmosphere. Still, it is coming out of an air-water system that will eventually, at some point after the removal process has been underway, reach a balanced condition. When that balanced condition is achieved by the air-water system the air will be transferring CO2 into the water at just the same rate as the intervention is taking it out of the water.
Refer to the DS and OG rates in Figure 3. The initial response to intervention will be an increase in the down-arrow DS value, to greater than 13 Gt, driven by the reduced CO2 partial pressure in the water. This will occur at the early stages, before the air has a chance to catch up with its own partial pressure reduction.
The up-arrow OG value will shrink for the same reason. Therefore initially the net transfer rate through the air-water interface will be greater than 10 Gt / yr.
As the years pass, with atmospheric CO2 concentration declining, air's partial pressure reduction will start catching up to water's reduction. The DS and OG arrows will both have smaller values (there is less CO2 in play), and their difference, DS - OG, will decline to less than 10 Gt / yr.
Here is an estimate of the time required to lower our current 397 ppm concentration to 350 ppm.
At 397 ppm, atmospheric CO2 content = 3.1 e18 grams, stated above. At 350 ppm it will be
3.1 e18 g x 350 / 397 = 2.7 e18 g CO2,
which implies an atmosphere aggregate reduction of 0.4 e18 grams (3.1 - 2.7 = 0.4).
It is not really correct to expect the ocean's aggregate CO2 reduction to be equal to the air's aggregate reduction, early into the intervention process (ocean is leading air, with air trying to catch up). But allow me to assume them equal anyway, for estimation purposes. That is, let the ocean’s CO2 reduction be set equal to 0.4 e18 grams, also .
This will have been accomplished when the total amount of CaCO3 that has been dissociated on land reaches
0.4 e18 grams CO2 x 100 g CaCO3 / 88 g CO2 = 0.45 e18 grams CaCO3.
proportional fraction
from above
From our mining calculations above, we anticipate dissociating 25 e18 grams per year of CaCO3, . Therefore the time to achieve 350 ppm is given by
time to 350 = 0.45 e18 grams CaCO3 / 25 e15 grams / year = 18 years
in order to return to 350 ppm via limestone intervention.
Through the years, as the system’s CO2 inventory declines, the extraction phenomenon is controlled by the inverse exponential relation. In that universal relation, all activity slows down because the rates of change are proportional to remaining quantities. But let me ignore that mathematical fact of nature, for purposes of rough estimation.
For atmospheric CO2 to reach the preindustrial 280 ppm, we can say
3.1 e18 grams x 280 ppm / 397 ppm = 2.2 e18 grams
of total CO2 in the preindustrial atmosphere
Thus we require an aggregate atmospheric CO2 reduction of 0.9 e18 grams (3.1 - 2.2 = 0.9).
CaCO3 consumption is given by
0.9 e18 g CO2 x 100 g / 88 g = 1.0 e18 grams CaCO3 consumed.
proportion
time to 280 ppm = 1.0 e18 grams CaCO3 / 25 e15 grams / year = 40 years
to return our atmosphere to 280 ppm.
To repeat, this 40-year result should not be taken literally. Since it ignores the exponential tapering effect, it is a rough order-of-magnitude estimate only.
All things considered, it’s an attractive option. I'm proposing to dig up, process, and distribute an additional 25 billion tonnes per year of limestone, on top of mankind’s current worldwide production of 20 billion tonnes. So total production must approximately double, which is surely doable. It needn't continue indefinitely; only until we get earth’s atmosphere back to normal.
_______________
* Regarding my high-hopes Time Frame assumptions stated earlier: Are they expecting too much?
Well, if we should find ourselves progressing slowly in shutting down petroleum and natural gas electric generation (assumption No. 1); or if the phasing out of petroleum transportation is proceeding sluggishly; or if the CCS goal that we set for ourselves regarding that 26 Gt CO2 from point-source emitters (assumption No. 2) fails to be reached; for any combination of these disappointments, we can attempt to compensate for our shortcomings by mining even more limestone than the 25 billion tonnes target called for by coal switchover. It’s not like there’s any shortage of limestone on earth.
_______________
How to get all that carbon-free energy
As already made clear, we'll need huge new supplies of thermal and electric energy over and above what modern societies are already consuming. Right off the bat, wind, water and solar cannot serve because they are low-temperature sources (assuming PV solar). So WWS are out of contention even before we bump into their intermittency and unreliability.
That leaves nuclear.
Liquid-Fuel Thorium Reactor - LFTR
There exists a nuclear fission technology, proved in concept but not yet developed to commercial status, that can furnish the energy needed for this intervention. And can do so without detriment to the ecosphere. It uses the element thorium for its fuel feedstock, rather than uranium. Its fuel is liquid, not solid as for all current reactors.
These two features, a) Liquid, and b) Thorium-based, confer stunning advantages which I will describe here briefly. For a thorough discussion of LFTR’s advantages in terms of 1) Operating safety; 2) Waste handling; 3) Long-term sustainability; and 4) Cost [in money, land-use, and CO2 footprint]. I invite you VEC judges to visit my websites www.dirkpublishing.com and www.timothymaloney.net . Within the forthcoming brief description, I will provide references to particular slideshow frames and blog postings which can be perused for more information.
A LFTR reactor uses thorium-232, which is an abundant element in earth's crust, to breed fissionable uranium-233 from. There is enough thorium on earth to power human civilization forever; we’ll never run out. The proof-of-concept experiment was performed at the US Oak Ridge National Laboratory - ORNL - in the 1960s and early 1970s. The research project was named the Molten Salt Reactor Experiment - MSRE. It was a complete success, conceptually.g But it was abandoned and never commercially developed. The reasons are immaterial.
Its design features and their relevance are here listed. I will speak in the present tense, as if their development were complete and LFTRs were actually in operation.
1) It operates with liquid fuel so there is no entrapment of contaminated solid-fuel pellets inside a sealed fuel-rod.h This is unlike a uranium light water reactor - LWR.
LFTR therefore achieves virtually 100% burn-up, compared to only 3% for a LWR. Thus it has virtually zero heavy-atom waste (uranium and plutonium).k Its medium-atom waste (fission-product waste) is simple to deal with.m
The fuel-containing liquid is continually replenished with thorium.p This is unlike a LWR which requires biannual refueling shutdowns for batch fuel-rod loading. LFTR never shuts down from the day that it goes critical until the day that it is decommissioned, a half-century later.
It’s fuel requirement is extremely small due to its 100% fission rate.It has about 200:1 advantage over LWR in that regard.q So it is a non-extractive technology and should be regarded as such.
It has zero CO2 footprint associated with isotope enrichment, unlike uranium ore. Thorium needs no isotope enrichment.r
2) It operates at a much higher temperature than LWR, which is only 300°C. The operating temperature for MSRE was 700°C.s Commercial LFTRs will operate in the 1000-1200 °C range.
Thus it achieves the temperature range needed for CaCO3 dissociation, and for synthetic fuel manufacture. Also its thermodynamic efficiency is much higher than LWR for electric generation.t
3) The reactor consists of low-pressure plumbing with a nuclear chain reaction occurring inside its pipes.v Its fluid pressure is less than at the kitchen faucet, so it won't spring a leak .
If for some reason we want to stop the fission reaction, we just allow the fuel liquid to flow out of the piping loop by gravity drainage.w The fuel flows and disperses into a wide dump tank where residual decay heat is a non-issue. Meltdown is impossible. There is nothing to melt because the fuel is already a liquid.
Because meltdown is impossible the reactor does not need: a) Strong containment vesselx;
b) Backup cooling system. It already didn't need a spent fuel (waste) storage pool, because it creates no spent fuel.
With so many LWR parts that are not needed, LFTR uses much less construction steel and concrete than LWR. It therefore has a smaller CO2 footprint for material. Even generation 3+ uranium LWR reactors are superior to wind and solar construction regarding steel, concrete, and CO2 footprint, by a factor better than 10:1y. Generation 4 LFTR will improve that advantage even further.
Informed opinion is that LFTR construction will cost less than $2 per watt of electric capacity.z That's even cheaper than coal.
My recommendation for LFTR technology has been presented on behalf of the extra energy that societies will need for intervening with limestone to support the ocean “machine” that's cleaning our atmosphere. That was the point of my proposal.
But LFTR can also replace existing coal-burners and natural gas-burners, thereby beating down that 33 Gt / yr EM value in Figure 3 that got us into this mess in the first place. Which was the requirement in assumption No. 1) at the beginning of the Time Frame section.
Synthetic transportation fuels
Electricity generation accounts for a large part of our worldwide 33 Gt / yr emissions, but transportation fuel, mostly gasoline and diesel, accounts for an equally large portion. Those hydrocarbon-derived fuels can be replaced with synthetic fuels, either for burning in internal combustion engines or for use in fuel-cell technology .
1) AMMONIA: The dominant internal combustion engine technology can be adapted to use ammonia, NH3, to be burned with air in a modified Otto- or diesel-cycle engine. The chemical reaction is
4NH3 + 3O2 —➤ 2N2 + 6H2O
air
with no carbon dioxide present. Ammonia can be carried in a vehicle’s fuel tank as either pressurized liquid or gas. In its liquid state it has about one half the energy density of gasoline.
Zero-carbon emission ammonia fuel can be manufactured by first electrolyzing water to produce gaseous hydrogen, H2. Then
3H2 + N2 —➤ 2NH3 which is compressed to a liquid to obtain portable fuel.
air 500 °C heat
from LFTR
The electric hydrolysis of water to obtain hydrogen gas H2 is illustrated in Figure 5.
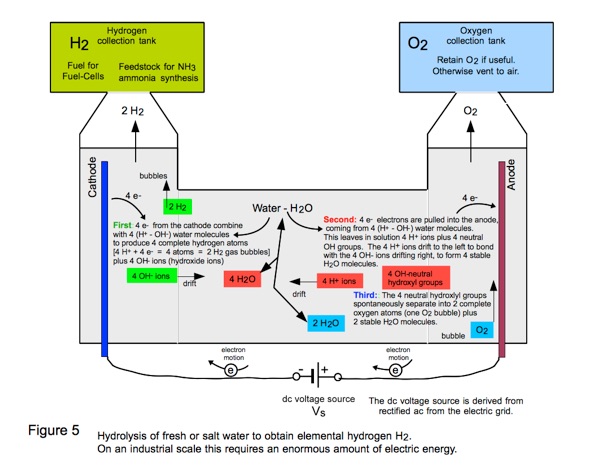
Thus LFTR furnishes both of the necessary energy inputs for ammonia manufacture: 1) Electric energy for splitting H2O to give H2; and 2) Heat energy to drive the bonding reaction of H2 to N2, nitrogen gas in the air. The entire process is carbon-free.
2) HYDROGEN FUEL-CELLS: Small and medium vehicles can be propelled by a hydrogen
fuel-cell operating an electric motor to turn the wheels. The fuel-cell takes in H2 gas from an
on-board hydrogen tank, and also takes in O2 oxygen from the air. Hydrogen atoms are ionized internally within the cell. That releases free charge-carriers, electrons, which move through an electric circuit to drive a dc electric motor connected to the cell's output terminals.
The fuel-cell acts like a dc battery, but it never discharges like a battery. It can run a vehicle's electric motor for as long as H2 gas is supplied at its input port, flowing from the vehicle's hydrogen fuel tank.
VEC judges, so ends my proposal for removing huge quantities of atmospheric CO2. The part played by humans terminates on a ship, slowly tracking its assigned route / pattern, with a screw-conveyor feeding a metered amount of calcium oxide into a discharge tube of adjustable depth.
During the first week the tube’s mouth is positioned 10 meters below the surface. During the second week the ship repeats its navigational pattern with the tube mouth held at 30-meter depth. The following week 50 meters deep, and so on.
The calcium fleet cruises 24 hours / 7 days. The system of resupply, whether done at sea by retrofitted oil tankers or by returning to port for dockside loading, will occupy our finest logistics experts, no doubt.
Thank you for your consideration.
Timothy J. Maloney
Reference Notes g through z were placed in the section Liquid-Fuel Thorium Reactor - LFTR, pointing to particular slideshow frames and blog postings which can be perused for more information.
In the list below, slide numbers refer to pages in the document Thorium Nuclear Slideshow which is downloadable from www.dirkpublishing.com, on page Slideshow downloads. It is also downloadable from www.timothymaloney.net, on page Thorium Energy.
Reference
Letter Slide Numbers
g 51, 52
h 26, 28, 46, 53, 82, 86
k 41, 43, 44, 63, 64, 71
m 41, 63-65, 84
p 82, 83, 86
q 59-62, 64, 85, 87
r 40, 60, 63
s 49, 62
t 61, 62, 84
v 48-50
w 55-57
x 26, 29, 30, 32, 34, 50, 53, 54, 57
y http://tinyurl.com/lsvq2yn Sept 2, 2013 @ www.timothymaloney.net
http://tinyurl.com/ntu5h3f Oct 7, 2014 @ www.timothymaloney.net
z 77, 81, 85, 87